Recently, the Insect Systematics and Diversity Team from our college has made new progress in the study of the coevolution of hemipteran insects and their symbiotic bacteria. The research results, titled "Symbionts in Hodgkinia-free cicadas and their implications for co-evolution between endosymbionts and host insects," were published in the American Microbiological Society's journal Applied and Environmental Microbiology, and were featured on the cover of the December 2023 issue. The first author of the paper is the graduate student Zhang Wenzhe, with Wang Jiali, Huang Zhi, and He Xiaohua as co-authors, and Professor Wei Cong as the corresponding author.
Hemipteran insects of the suborder Auchenorrhyncha have a typical piercing-sucking mouthpart and feed on plant juices rich in carbohydrates but lack of nitrogen nutrients. Therefore, these insects must rely on intracellular symbiotic bacteria to provide essential amino acids, riboflavin, and other nutrients missing from their diet.
Previous studies have shown that Cicadidae insects, a family of hemipteran insects, contain two obligate symbiotic bacteria: Candidatus Sulcia muelleri (short as Sulcia) from the Bacteroidota and Candidatus Hodgkinia cicadicola (short as Hodgkinia) from the α-Proteobacteria. However, during long-term evolution, some Cicadidae insects have been found to have a yeast-like fungal symbiont (short as YLS) that replaces Hodgkinia. In Hodgkinia-free cicadas, Sulcia is found in the mycetocytes of adults, while the YLS is only found in the fat body due to its lower adaptability. However, recent studies have shown that YLS is also present in the mycetocytes of adults in Hyalessa maculaticollis (the Sonatini tribe) and Graptopsaltria tienta and G. nigrofuscata (the Polyneuriini tribe).
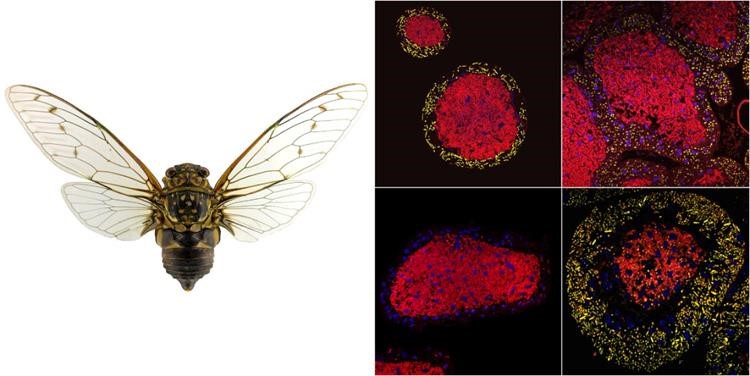
Figure 1 Composition and distribution of symbiotic bacteria in the M. punctulatus and its bacterial reservoir
The research team conducted a systematic study of the symbiotic bacteria of the Sonatini and Polyneuriini tribes and discovered that YLS is commonly found in mycetocytes of these adults (Figure 1). The mycetocytes, which were originally important immune organs similar to the fat body in Auchenorrhyncha insects, have gradually evolved into symbiont-containing organs specialized for accommodating obligate symbiotic bacteria during long-term evolution. Although the genome of Sulcia is conserved and stable, its 16S rRNA gene has undergone mutations in some groups such as the Sonatini tribe (Figure 2 top). The YLS, which is believed to have evolved from pathogenic fungi of the genus Ophiocordyceps, has a phylogeny that is consistent with the host only in some groups but is significantly different overall, indicating that YLS infected different ancestral lineages of Cicadidae insects during evolution and subsequently underwent further differentiation. During the evolution of Cicadidae insects, YLS has replaced Hodgkinia and YLS. The replacements have occurred repeatedly (at least 6 times for the first one and 7 times for the second one) (Figure 2 bottom), which should be related to their long-term underground lifestyle (up to more than ten years) and their very short adult stage (only a few weeks). The soil environment facilitates their repeated infections and recruitment of microorganisms, allowing them to establish symbiotic relationships with associated microorganisms. This study provides new information for further understanding the coevolution of piercing-sucking mouthpart insects with their symbiotic bacteria in different habitats and ecological niches.
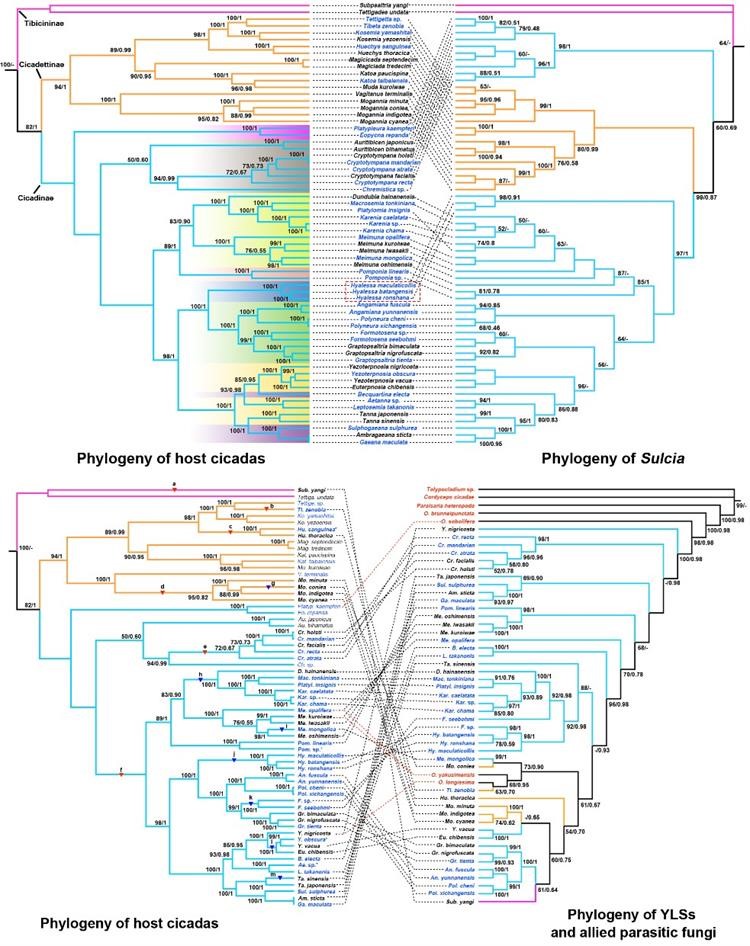
Figure 2 Comparison of phylogenetic relationships between host insects and symbiotic bacteria Suclia (top) and YLS (bottom)
This research was funded by the National Natural Science Foundation of China (Grant numbers 32270496, 32070476).
Original link: https://journals.asm.org/doi/10.1128/aem.01373-23